Isolation and selection of Endophytes and Biocontrol Agents (EBCA)
Task 2.1 EBCAs of Fusarium oxysporumf. sp. cubense
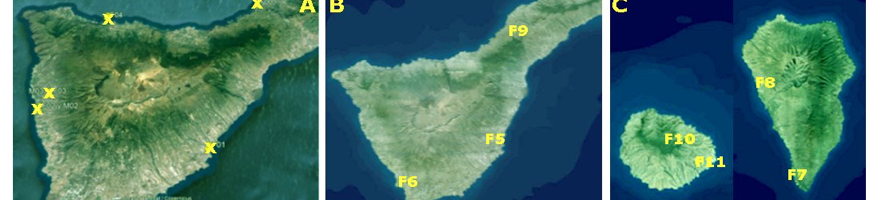
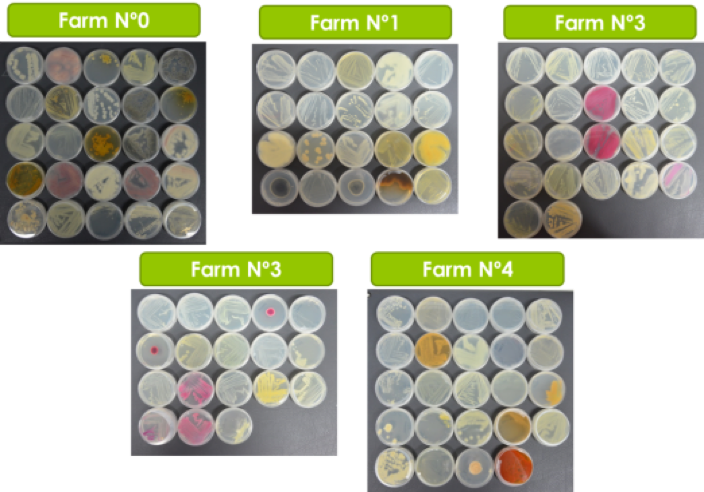
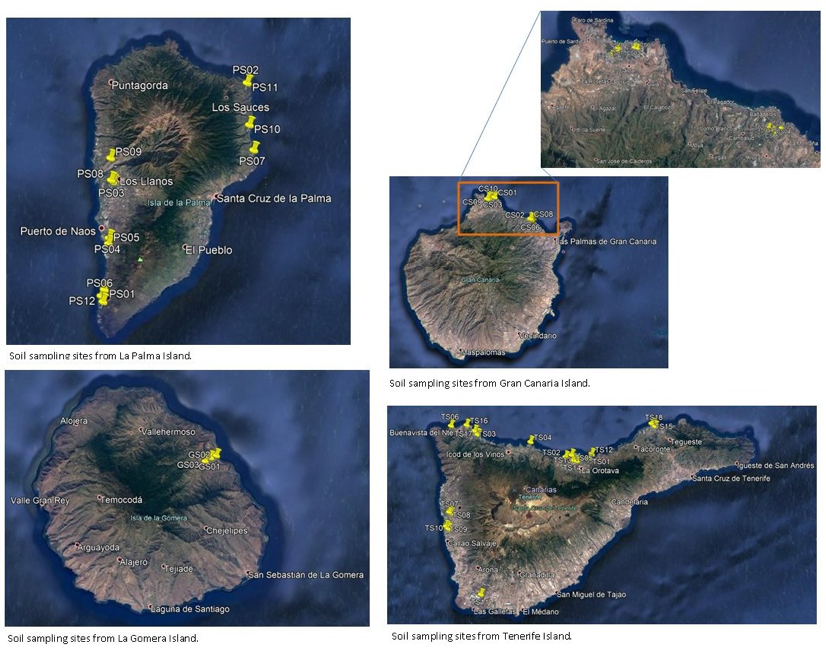
A survey was performed by IAS CSIC with Partner 4 Coplaca (Dr. Ing. Javier López-Cepero, Fig. 2), to identify and collect suitable EBCAs from farms (Fig. 1A, Table 1) at Tenerife Island.
| Farm Nº | Farm name | Lat. (N) | Long. (W) | Altitude (msl) |
| F00 | Escuela Capataces | 28°29’46” | 16°25’15” | 299 |
| F01 | Siverio | 28°10’06” | 16°26’13” | 37 |
| F02 | Temaso | 28°09’00” | 16°47’36” | 84 |
| M02 | Temaso | 28°09’00” | 16°47’36” | 84 |
| F03 | Servicios Agrícolas Abdul | 28°11’02 | 16°47’01” | 326 |
| M03 | Servicios Agrícolas Abdul | 28°10’60” | 16°47’02” | 306 |
| F04 | Malpaís – Colpon Agrícola | 28°22’36” | 16°44’08” | 22 |
Table 1. Characteristics of farms sampled in Tenerife by IAS-CSIC and Coplaca (first round).
A second sampling (in collaboration with Partner 4, Coplaca; Personnel involved, Dr. Ing. Javier López-Cepero) was carried out in new farms from seven farms in (Fig. 1 B, C and Table 2) different banana orchards at Tenerife, La Palma and La Gomera.

| Farm | Farm name | Latitude (N) | Longitude (W) | Altitude (msl) |
| F05 | Fco Pacheco, Arico, Tenerife | 28°10’67” | 16°27’64” | 188,64 |
| F06 | La Caldera, Adeje, Tenerife | 28°04’84” | 16°43’32” | 113,22 |
| F07 | Siso, FuencalienteLa Palma | 28°28’97” | 17°52’58” | 28,77 |
| F08 | Ortiz, Tijarafe, La Palma | 28°41’37” | 17°57’01” | 299,56 |
| F09 | Escuela Capataces, Tenerife | 28°29’46” | 16°25’15” | 299 |
| F10 | Hermigua, La Gomera | 28°10’82” | 17°11’08” | 123,15 |
| F11 | David, San Sebastián, La Gomera | 28°06’69” | 17°08’68” | 82,82 |
Table 2. Farms surveyed in Tenerife, La Palma and Gomera islands by partner 5 (IAS-CSIC),in collaboration with Partner 4.
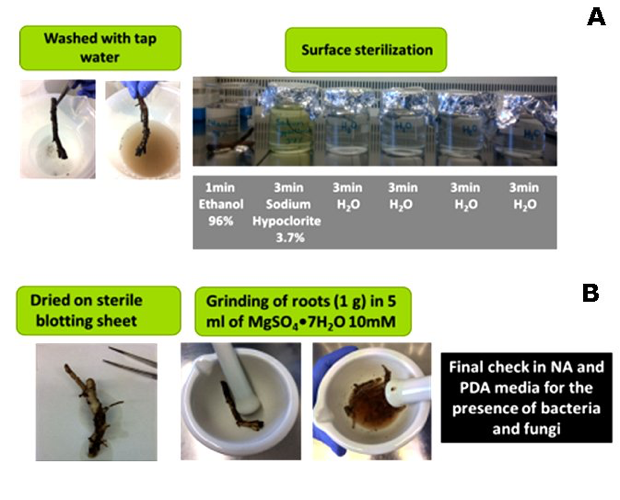
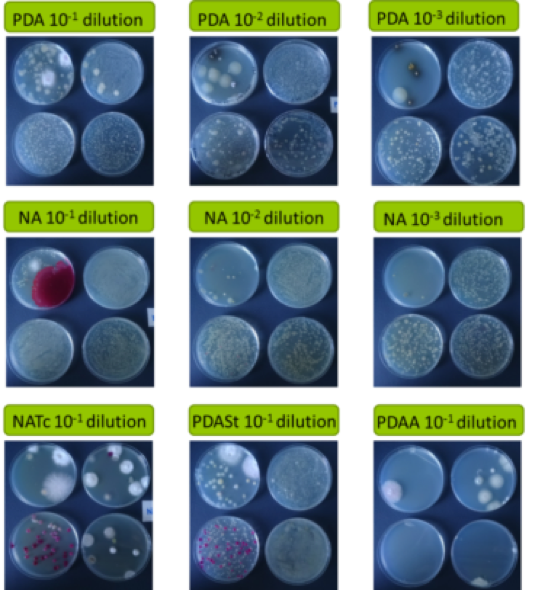
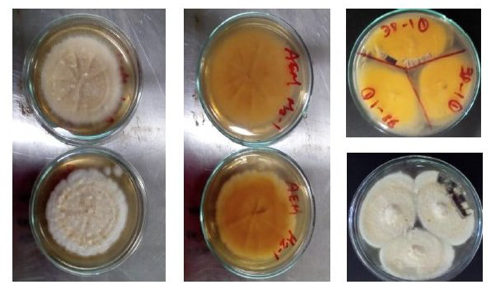
Rhizosphere soil samples were transferred to Partner 1 (CNR, Bari, IT) for further isolations. Surface sterilization was carried out (Fig. 3B) testing five different media withad hocdilutions (Fig. 4), observed daily to check for bacterial/fungal growth. Single colonies were transferred to PDA and selected according to speed of growth and morphology (colour, size, shape) (Fig. 5). Pure cultures from single colonies were grown in Luria Bertani (LB, bacteria) or PDA (for fungi) and cryopreserved at -80ºC until use. 696 single/pure bacteria and 162 fungi isolates were obtained from the first sampling.
In vitro antagonism assays. Bacterial and fungal isolates were tested against a representative isolates of Foc subtropical race 4 (STR4), race 1/2 (R1/R2)(isolates CAV-095 and CAV-2790, kindly provided Prof. Altus Viljoen, Stellenbosch Univ., South Africa) and TR4 (isolate II5, kindly Prof. Antoni Di Pietro, Córdoba University, Spain), causal agents of PD.To determine the in vitro antagonist activity of root endophytes, dual cultures were used with CAV-095 (mycelium and conidia) and endophytic bacteria or fungi inoculated at four equidistant points from the pathogen spot (Fig. 6). One control plate with the pathogen alone was included. Currently, bacteria and fungi isolated from the second sampling are being tested against CAV-095 (STR4) and a representative isolate of Foc tropical race 4 (TR4) following the same protocol.
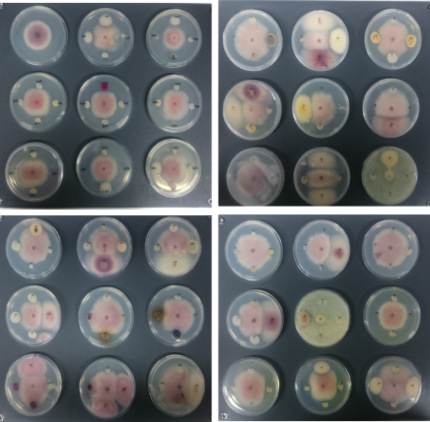
More than 100 strains showed in vitroantagonism against Foc CAV-095 at a variable degree, showing that banana roots are a rich reservoir of beneficial endophytic antagonists. 88 strains (first sampling) with best potential as EBCAs were selectedanddual cultures were performed with Foc TR4 (mycelium plus conidia). Antagonist activity (i.e., halos or inhibition zones) was then scored.
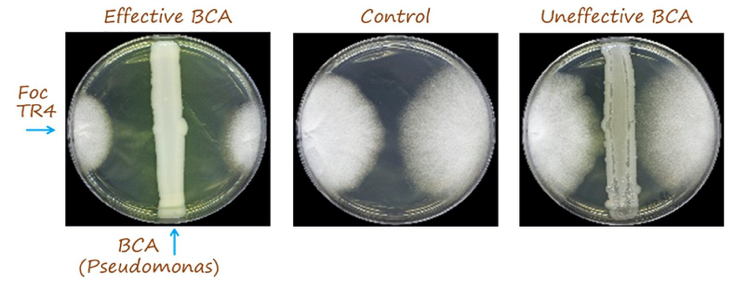
The relative inhibition index was calculated according to the equation (Rc-Ra)/Rc, where Rcis the average radius of FocTR4 colony in the absence of antagonist microorganism and Rais the average radius of FocTR4 colony in the presence of antagonist microorganism (four equidistant points). Differences were found depending on the culture medium used (PDA or NA).Inhibition of Foc growth was more frequent in NA. Twenty six and nine selected endophytes significantly inhibited growth of Foc TR4 (relative inhibition >0.30) in NA and PDA media, respectively. Isolate #793 (molecularly identified as Pseudomonas protegens; see below) was the most promising strain showing a relative inhibition index of 0.43 (PDA). Based on the results obtained from in vitrotests in NA medium, the three most promising strains were #102, #248 and #301 (molecularly identified as Pseudomonas chlororaphisthe first and Pseudomonasspp.). A relative inhibition index >0.60 was measured for these three Pseudomonas spp. isolates (Fig.7).
Phenotype characterization.Assays were performed to check phenotypes associated with biocontrol and/or plant growth promotion such as volatile production (e.g. 2,3 butanediol and hydrogen cyanide), enzymatic activities such as catalase, xylanase, amylase, phytase, protease and β-glucosidase, and siderophore production (Table 3).
| Phenotype | Number of isolates | Frequency (%) |
| Phytase | 55 | 62.5 |
| Protease | 51 | 57.9 |
| Xylanase | 6 | 6.8 |
| Catalase | 84 | 95.4 |
| Siderophores | 67 | 76.1 |
| Amylase | 19 | 21.6 |
| b-Glucosidase | 35 | 39.8 |
| 2,3 Butanediol | 16 | 18.2 |
| HCN | 30 | 34.1 |
Table 3. Evaluation of phenotypes associated with biocontrol and/or plant growth promotion.
Molecular identification.The 88 strains, selected at CSIC according to their Focantagonistic effect,are currently being identified at the molecular level by sequencing 16S rDNA and gyrBgenes for bacteria, andTranslation Elongation Factor 1-alpha (tef1) and Internal Transcribed Spacer (ITS) for fungi. Data showed a low diversity among bacteria, Pseudomonasbeing the most predominant. Fungi showed higher diversity, with genera Gloeotinia, Fusarium, Plectosphaerella, Gliocladium, Epiccocumand Acremonium.
CNRisolated Bacillusand Streptomycesspp. and other EBCAs from banana rhizosphere, selected for in vitroantagonism against Foc, producing a collection at IPSP CNR (Bari, IT), tested for efficacy against PD in pot-grown banana. Samples proceeded from the Oct. 2017 survey carried out by Coplaca and IAS CSIC (43 soil samples), and from a second survey carried out on Feb. 2018, in collaboration with Coplaca, aiming at nematode antagonists.
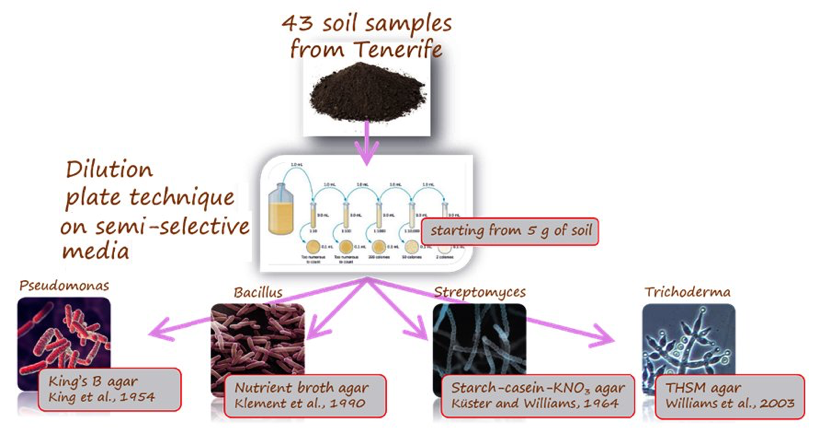
Isolation of EBCAs from banana rhizosphere.EBCAs were isolated from soil onto agar media according to the dilution plate technique(Fig. 8). A total of 516 plates were used. Colonies of Bacillusand Pseudomonasspp. were visible one-two days after plating, and of Streptomycesand Trichodermaspp. 5-7 days after plating, identified by the colony morphology (Fig. 9).
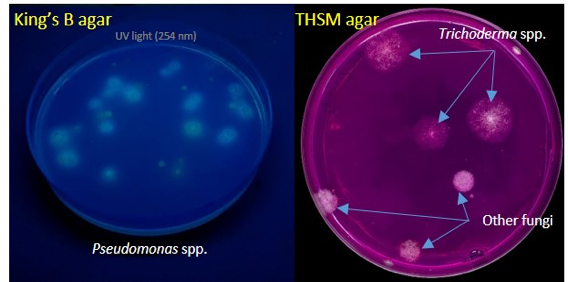
Figure 9.Isolation of microorganisms from soil by the dilution plate technique. King’s B agar plate with colonies of Pseudomonasspp. showing fluorescence under UV light at 254 nm (left). THSM agar plate with colonies of Trichodermaspp. (right).
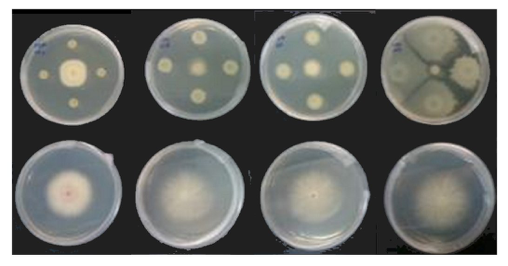
Selection of EBCAs for in vitro Focantagonism. EBCAs were screened in vitroat CNR for their antagonistic activity against Foc race 1 (Foc R1) and Foc tropical race 4 (Foc TR4) by the dual culture method (Fig. 10), keeping the isolates developing an inhibition halo vs Foc.
Establishment of collection. A total of 516 plates were used for EBCAs isolation. Because antagonism against Foc was a priority, promising antagonists were selected regardless of taxonomic classification. About 1000 isolates of Bacillusand Streptomycesspp. and over 340 isolates belonging to Pseudomonasspp. and Trichodermaspp. were screened for antagonism against Foc R1 and Foc TR4.Finally, a total of 87 isolates were selected as Foc antagonists and stored in collection at IPSP CNR.
| Taxon | Isolates |
| Pseudomonas sp. | P1C1, PS5, P1A1, PS3.1, P3E1, PS6, P4AOD1, P2D1, P4A1, P2B1, P2C1.2.1 |
| Bacillus sp. | BT1, BS6, B2C1.2.1, BN8.1, BS7, B4C1, B2C1.2, B1A1, BN8.2, B4B1.2, B2AOB1 |
| Streptomyces sp. | St2AOB1, St3B1.1, St4C1, St3A1, St2C1.1, St2B1, St3D1, St4AOD1, St1D1, St2A1.2, St3C1 |
| Trichoderma sp. | T2C1.4, T2A1.1, T2A1.2, T2B1.1, T2B1.2, T2C1.1, T2C1.2, T2C1.3, T2E1, T3A1.2, TS1 |
Table 4.Initial composition of the SynCom 1.0 (first in planta assay, 44 isolates).
Evaluation of selected EBCAs against PD in pot-grown banana.Most effective 44 isolates were used to set up a synthetic microbial community (SynCom, Table 4), tested on banana plants cv. Gran Enana in pots with sterilized soil, inoculated with known concentrations of FocTR4 with/without added SynCom, and incubated in a conditioned quarantine greenhouse. PD symptoms were monitored periodically as well as the soil dynamics of Foc and SynCom. In a first assay, PD symptoms appeared 14 days post-inoculation (dpi) and increased afterwards. Inoculation with SynCom reduced the symptom severity of PD (Fig. 11), although not affecting its prevalence (percent of diseased plants).
The SynCom population components remained viable and active throughout the experiment and were compatible (not deleterious to) the Gran Enana roots. The pathogen impacted negatively on the chlorophyll content of leaves, an effect that the SynCom mitigated (Fig. 11). The results showed that SynCom reduced partially the PD severity, probably by indirect mechanisms, because no pathogen regulation was detected in soil. These results were confirmed in a second independent assay with a reduced SynCom (Table 5).

| Taxon | Strains |
| Pseudomonassp. | P1C1, PS5, P1A1 |
| Bacillus sp. | BT1,BN8.2 |
| Streptomycessp. | St2AOB1 |
| Trichodermasp. | T2C1.4 |
Table 5.Composition of the reduced SynCom_1.1 (second in plantaassay, 7 isolates).
A third experiment (III), was carried out to check if the SynCom could reduce the Foc TR4 inoculum in soil. Three batches of soil were prepared in triplicate with pathogen concentrations ranging from 103to 107cfu g-1soil, with or without SynCom. Again, the FocTR4population, monitored periodically, was not regulated, regardless of the SynCom concentration.
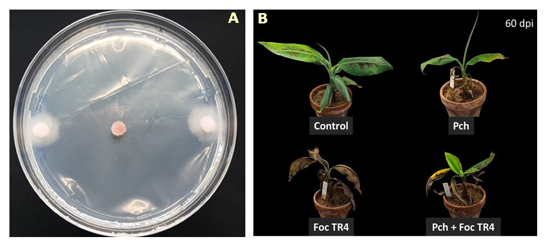
In a forth assay, the isolate of Pochonia chlamydosporiaDSM 26985 (commercialized by SacomLab)was tested in plantafor control of Foc TR4. Such isolate resulted effective in vitroagainst the pathogen (Fig. 12A), when a conidial suspension was inoculated in pot-grown plants. One week later, plants were transplanted in soil artificially inoculated with 104cfu g-1of Foc TR4. Plants treated with DSM 26985showed less disease symptoms at early stages of PD development (35 dpi), compared to Foc TR4-inocualted control. However, as the disease progressed, difference between treated and untreated plants disappeared, and became undetectable at 60 dpi (Fig. 12B).
Isolation of beneficial EBCAs in SSA. Soil bacteria and fungi for PD control were isolated in Kenya by Real IPM, IITA and ICIPE. Isolation is still ongoing for screening in greenhouse and field conditions. In Tanzania, 370 bacterial isolates were obtained from 35 corms and roots of Mchare type banana plants with or without PD symptoms from Arusha and Kilimanjaro. Isolation was carried out according to the protocol developed by IAS CSIC and shared through the MUSA project. Pure cultures isolates are under long-term storage in glycerol at -80°C. Sampling was conducted twice, in Jan. 2018 (summer) and July 2018 (winter,dry season). Of the 370 potential bacterial endophytes obtained, 131 proceeded from corms and 239 from roots. The isolates were characterized with morphology (colony shape, color) and Gram staining tests. DNA was extracted from all 370 isolates, the 16S rRNA gene amplified and sequenced for molecular identification.
In Uganda,a total of 534 bacterial and 56 fungal isolates were produced by IITA and characterized using morphology (colony shape, and color) and Gram staining. Sampling for mycotoxin analysis was conducted twice. The first was done in March, 2018 for samples collected from cultivars Silk (AAA) and Sukali Ndizi (AAB), in Kawanda. As in Tanzania, samples were collected from plants with or without PD symptoms at the same physiological maturity. The site was selected for the semi-controlled infection conditions that guaranteed the presence of the pathogen in both symptomatic and asymptomatic plants.
Scientists at ICIPE,IITA and Real IPM collaborated in Kenya for assessment of antagonists against BW and PPN (Table 6). They could also recover a Beauveria bassianastrain first isolated by NARO (Caroline Nankinga) from weevils in Uganda and used previously by both NARO and IITA, which performed efficiently in the field. As it could not be found in Uganda but it had been deposited with CABI, it was recovered from this collection and is now in use.
The Foc endophyte V5W2 previously isolated by IITA demonstrated to provide good management of nematodes, with a growth stimulus. These two isolates were used to assess their efficacy in isolation as well as combined, under field conditions. Scientists from IITA, ICIPE, Real IPM and KU Leuven selected isolates by: i)leveraging on other/previous projects that have bioprospected microbes – such as the 1500 populations isolated under the banana Xanthomonas wilt (BXW) project (the best five populations that worked against BXW were selected for assessment in MUSA); ii)bioprospecting sites with Fusarium problems and isolating new popualtions for in vitroand in vivoassessment (molecular tools/identifications are applied to focus on which populations to select), andiii)assessing best populations from institutional collections (ICIPE/RealIPM) including those already marketed as products, unsing in addition a F. oxysporumand a B. bassianapopulations that worked well on banana and that were taken to the field to maximise assessment of previously proven populations.
| Isolate code | Taxonomic name | Repository /Institute | Isolation Source | Isolation site | Target organism(s) |
| ICIPE 284 | Beauveria bassiana | ICIPE | Soil | Mauritius | BW |
| ICIPE 648 | B. bassiana | ICIPE | BW | ||
| ICIPE 647 | B. bassiana | ICIPE | Soil | Mauritius | BW |
| ICIPE 662 | B. bassiana | ICIPE | BW | ||
| ICIPE 660 | B. bassiana | ICIPE | BW | ||
| ICIPE 609 | B. bassiana | ICIPE | BW | ||
| ICIPE 273 | B. bassiana | ICIPE | Soil | Mbita (Kenya) | BW |
| ICIPE 281 | B. bassiana | ICIPE | Soil | Mauritius | BW |
| ICIPE 622 | B. bassiana | ICIPE | BW | ||
| ICIPE 644 | B. bassiana | ICIPE | BW | ||
| ICIPE 69 | Metarhizium anisopliae | ICIPE | Soil | Matete (DRC) | BW |
| ICIPE 62 | M. anisopliae | ICIPE | Soil | Matete (DRC) | BW |
| ICIPE 78 | M. anisopliae | ICIPE | Temnoschoita nigroplagiata | Ungoye (Kenya) | BW |
| ICIPE 682 | Isaria sp. | ICIPE | BW, PPN | ||
| ICIPE 700 | Trichoderma asperellum | ICIPE | BW, PPN | ||
| ICIPE 710 | Trichoderma sp. | ICIPE | BW, PPN | ||
| ICIPE 712 | Fusarium sp. | ICIPE | BW, PPN | ||
| ICIPE 697 | Hypocrea sp. | ICIPE | BW, PPN | ||
| ICIPE 279 | B. bassiana | ICIPE | Coleopteran larvae | Kericho (Kenya) | BW |
| ICIPE 621 | B. bassiana | ICIPE | BW | ||
| ICIPE 620 | B. bassiana | ICIPE | BW | ||
| BW KM | Beauveria sp. | ICIPE | C. sordidus | Kalro, Mwea, Kenya | BW |
| SD 228 | T.asperellum | Real IPM | Kenya | PPN | |
| SD 298 | T.hamatum | Real IPM | Kenya | PPN | |
| SD 229 | B.bassiana | Real IPM | Kenya | BW | |
| SD 277 | B. bassiana | Real IPM | Kenya | BW | |
| V2W2 | Fusarium oxysporum | IITA | Banana roots | Uganda | BW, PPN |
| V5W2 | F. oxysporum | IITA | Banana roots | Uganda | BW, PPN |
| Bb WA | B. bassiana | CABI (NARO ) | C. sordidus | Uganda | BW, PPN |
Table 6. Antagonists tested in Kenya against banana weevils and nematodes.
In Luwero, IITA scientists selected five symptomatic and five asymptomatic banana plants of the cultivar Sukali Ndizi (AAB). This field was selected because plants were embedded in an agroforestry ecosystem with coffee and cassava plants that might influence higher variability in the endophyte populations. In a third site, Kisoga, 5 symptomatic and 5 asymptomatic plants of cultivar Sukali Ndizi were selected.
Task 2.2 – EBCAs for banana and enset PPNs
Samples collected in Tenerife (Feb. 2018) by CNR showed a 77% prevalence of Pratylenchus goodeyi, either in northern and southern farms. Helicotylenchusspp. were also detected, with 81% prevalence, including H. multicinctusand H. abunaami. Nematode antagonists isolated from soil included Drechslerella dactyloides, Lecanicilliumsp. (likelyL. psalliotae,identification with ITS sequencing), and othen generalist nematode trapping fungi, under evaluation with SacomLab. The nematode populations are currently multiplied on Gran Enana plants in greenhouse conditions. A Pasteurian. sp. population, available in collection at IPSP Bari and parasitic on Helicotylenchusspp., is under evaluation for parasitism of H. multicinctusand possible culturing.
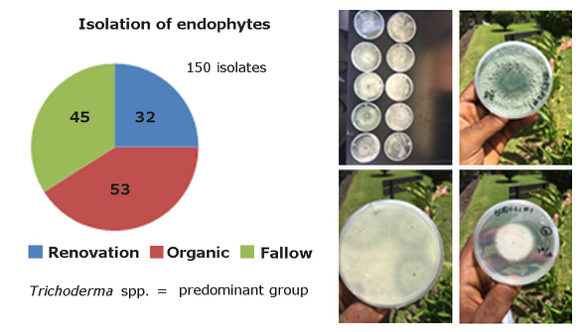
For isolation and selection of EBCAs from Central America, activities were carried out at EARTH University in conventional renovation, fallow and organic production systems. A study aimed at selection of best endophytes isolated by scrrenings in greenhouse conditions. A total of 150 endophytic fungi were collected in the three systems, with 53 from organic banana systems, 45 from fallow and 32 from conventional renovation (Fig. 13). The frequency of root colonization by endophytes was higher in organic and fallow rather than conventional renovation. The population of Radopholus similiswas singnifically higher in the latter, in comparison to organic and fallow systems. All fungi are stored in the laboratory collection of plant pathology of EARTH University.
The interaction between plants and selected EBCAs was evaluated by EARTH. A total of nine isolates of Trichodermaspp. were evaluated in the greenhouse on tissue-cultured plants of Grande Naine (AAA) for evaluation of growth promotion effects. The best isolates selected for plant growth promotion were Endo 1, 2 and 3 from organic banana system and Endo 4 from fallow. Currently, all the four isolates are evalauted for biological control activity against R. similisin greenhouse conditions. In addition, the four endohytes were inoculated in a test banana field for starting preliminary field assays against PPN.
Three isolates with potential for PPN biomanagement were collected from sampling sites in Kenya by Real IPM, preliminarly identified within the biopesticides R&D laboratories, and then based on gene sequencing (performed at CABI, UK).
Several surveys were carried out by CENSA for new isolates of Pochonia, Trichoderma, Beauveria and entomopathogenic nematodes (EPN) from banana/plantain soils, in different provinces of Cuba, in areas of low inputs or subsistence agriculture, in fields that did not previously receive applications of biological control agents.

For isolates of Pochonia spp. a dilution plating technique on semi-selective medium was used. After day 21, typical colonies of Pochonia spp. were grown on malt agar extract. A total of 36 isolates were identified as P. chlamydosporia, 15 proceeding from roots and 3 as endophytes (Fig. 15).
Out of 59 analyzed samples, proceeding from the rhizosphere of plantain and banana in the different provinces, 20 were assigned to Trichoderma,5 of which as endophytes. The colonies showed differences in texture and color at 72 hrs (Fig. 16). UA identified fungal EBCAs from CENSA through molecular methods, by PCR with specific primers (ITS).

Task. 2.3 Antagonists and EBCAs of BW (
UA implemented methods for isolation of entomopathogenic fungi (EF) from Canary Islands soils from banana plantations, with wide potential to be used as BW antagonists. In total 43 samples were collected (Fig. 17) in collaboration with Partner 4 Coplaca (Dr. Javier Lopez-Cepero): 3 from La Gomera, 18 from Tenerife, 10 from Gran Canaria and 12 from La Palma (Table 7) islands, sent to the Plant Pathology Lab. at the University of Alicante for EF isolation.

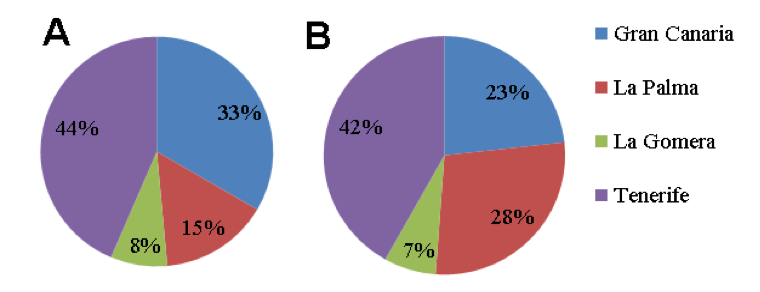
Collection of soils from banana plantations. Soil sampling (Table 1) was performed in Canary Islands in which banana is the major crop. Number of samples collected per island was proportional to the area devoted to banana cultivation (Fig. 18). Most soil samples (18 out of 43) were taken from Tenerife, the largest island with banana as major crop, at all altitude ranges. Soil samples were also taken from fields under sprinkler and drip irrigation (Table 7), from plantations under all crop management regimes, including ECO (plantation fields under organic production, EU Rules 834/2007 and 889/2008), with low environmental impact and fields with integrated production regimes (UNEGAP / Global Gap Rule) with a reduced use of chemicals. Finally, conventional cultivation included traditional crop management with chemical products (eg. pesticides and fertilizers). According to coordinates, soil sampling covered most of crops orientations (Fig. 18).
Detection and isolation of entomopathogenic fungi (EF) Galleria mellonella was used as living bait for EF isolation, with larvae (L3-L4) incubated at 25 °C for 15 days, to favour contact with soil (Fig. 19).
| Date | Island* | Sample | SIGPAC Lat. Long. | Alt. (m) | Irrigation | Crop Certification** | ||
| 04/07/2017 | G | GS01 | 38/21/16/9006/2 28°10’24″N 17°11’17″W | 82 | Sprinkler | U | ||
| 04/07/2017 | G | GS02 | 38/21/15/13/2 | 28°10’38″N | 17°11’00″W | 32 | Sprinkler | U |
| 04/07/2017 | G | GS03 | 38/21/15/167/1 28°10’07″N 17°11’31″W | 83 | Sprinkler | U | ||
| 31/10/2017 | P | PS01 | 38/14/21/64/1 | 28°30’06N | 17°52’16″W | 56 | Sprinkler | P.Int |
| 31/10/2017 | P | PS02 | 38/7/6/306/1 28°49’49″N 17°47’00″W | 170 | Sprinkler | Conv | ||
| 31/10/2017 | P | PS03 | 38/24/13/218/1 | 28°38’26″N | 17°55’09″W | 267 | Sprinkler | ECO |
| 31/10/2017 | P | PS04 | 38/24/26/10/1 28°34’06″N 17°53’36″W | 69 | Sprinkler | Conv | ||
| 31/10/2017 | P | PS05 | 38/24/26/82/1 | 28°33’40″N | 17°53’27″W | 34 | Sprinkler | Conv |
| 02/01/2018 | P | PS06 | 38/14/22/97/4 28°29’31″N 17°52’20″W | 10 | Sprinkler | Conv | ||
| 06/02/2018 | P | PS07 | 38/30/7/156/1 | 28°44’58″N | 17°44’11″W | 185 | Sprinkler | ECO |
| 08/02/2018 | P | PS08 | 38/24/13/56/1 28°38’31″N 17°55’21″W | 250 | Sprinkler | Conv | ||
| 09/02/2018 | P | PS09 | 38/47/13/560/1 | 28°40’09″N | 17°56’08″W | 360 | Sprinkler | Conv |
| 09/02/2018 | P | PS10 | 38/30/13/252/1 28°46’44”N 17°45’24” W | 70 | Sprinkler | Conv | ||
| 09/02/2018 | P | PS11 | 38/7/6/306/1 | 28°49’49” N | 17°46’58” W | 160 | Sprinkler | Conv |
| 09/02/2018 | P | PS12 | 38/14/10/36/2 28°29’08” N 17°51’59” W | 63 | Sprinkler | Conv | ||
| 20/07/2017 | T | TS01 | 38/26/3/48/ 1 a 6 | 28°24’23″N | 16°30’50″W | 215 | Drip | P.Int |
| 04/08/2017 | T | TS02 | 38/31/9/181/2 28°23’28″N 16°34’18″W | 230 | Drip | No | ||
| 09/08/2017 | T | TS03 | 38/42/1/32/1 | 28°22’23″N | 16°48’51″W | 50 | Drip | P.Int |
| 09/08/2017 | T | TS04 | 38/18/3/403/2 a 5 28°23’41″N 16°40’12″W | 115 | Drip | P.Int | ||
| 09/08/2017 | T | TS05 | 38/28/5/9065/9 | 28°24’00″N | 16°33’34″W | 150 | Drip | P.Int |
| 10/08/2017 | T | TS06 | 38/10/5/17/3 28°21’53″N 16°52’32″W | 60 | Drip | ECO | ||
| 20/09/2017 | T | TS07 | 38/19/8/9000/136 | 28°10’48″N | 16°47’47″W | 170 | Drip | ECO |
| 20/09/2017 | T | TS08 | 38/19/7/34/8 28°10’52″N 16°48’02″W | 135 | Drip | P.Int | ||
| 20/09/2017 | T | TS09 | 38/1/1/82/1 | 28°08’54″N | 16°47’43″W | 70 | Drip | P.Int |
| 20/09/2017 | T | TS10 | 38/10/51/4 28°08’42″N 16°47’16″W | 80 | Drip | P.Int | ||
| 20/09/2017 | T | TS11 | 38/6/4/214/6 | 28°01’56″N | 16°38’58″W | 75 | Sprinkler | P.Int |
| 09/01/2018 | T | TS12 | 38/26/1/57/6 28°25’04″N 16°30’43″W | 100 | Drip | Conv | ||
| 09/01/2018 | T | TS13 | 38/26/8/43/1 | 28°23’29″N | 16°32’30″W | 91 | Drip | Conv |
| 09/01/2018 | T | TS14 | 38/26/9/91/2 28°23’24″N 16°33’04″W | 252 | Drip | Conv | ||
| 09/01/2018 | T | TS15 | 38/23/1/170/1 | 28°31’51″N | 16°23’47″W | 75 | Drip | Conv |
| 09/01/2018 | T | TS16 | 38/10/1/146/10 28°22’44″N 16°50’20″W | 95 | Drip | Conv | ||
| 09/03/2018 | T | TS17 | 38/42/7/6/16 | 28°22’04” N | 16°48’13” W | 72 | Drip | ECO |
| 12/03/2018 | T | TS18 | 38/23/2/44/3 28°31’38″N 16°23’09” W | 115 | Drip | Conv | ||
| 13/03/2018 | C | CS01 | 35/09/2/111 | 28°09’28″N | 15°39’01” W | 75 | Drip | Conv |
| 13/03/2018 | C | CS02 | 35/6/6/1/1 28°08’05” N 15°31’36” W | 145 | Drip | Conv | ||
| 13/03/2018 | C | CS03 | 35/9/2/103/2 | 28°09’27” N | 15°39’11” W | 50 | Drip | Conv |
| 13/03/2018 | C | CS04 | 35/6/6/10/1 28°08’01” N 15°31’14” W | 180 | Drip | Conv | ||
| 13/03/2018 | C | CS05 | 35/6/8/272/1 | 28°08’24” N | 15°31’03” W | 135 | Drip | Conv |
| 13/03/2018 | C | CS06 | 35/6/8/455/3 28°08’10” N 15°31’23” W | 135 | Drip | Conv | ||
| 13/03/2018 | C | CS07 | 35/9/1/244/1 | 28°09’11” N | 15°39’58” W | 17 | Drip | Conv |
| 13/03/2018 | C | CS08 | 35/6/7/312/1 28°08’14” N 15°31’36” W | 135 | Drip | Conv | ||
| 13/03/2018 | C | CS09 | 35/9/1/260/4 | 28°08’49” N | 15°40’07” W | 32 | Drip | Conv |
| 13/03/2018 | C | CS10 | 35/9/1/288/1 28°09’03” N 15°39’52” W | 40 | Drip | Conv |
Table 7. Soil sampling data from banana fields in the Canary Islands.
* G=La Gomera, P=La Palma, T=Tenerife, C=Gran Canaria. **P. Crop type: Int=Integrated, ECO=Ecological, Conv=Conventional, U=unknown.

After 15 days incubation, insects recovered from soil were surface sterilized, dried and placed in moist chambers at 25°C in the dark, to allow growth and sporulation of colonising fungi. Larvae were then plated on corn meal agar (CMA) supplemented with antibiotics and Triton X-100. Fungi present on the larvae were isolated on CMA (Fig. 20).
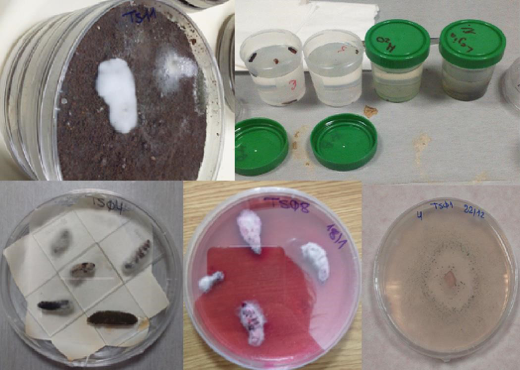
Isolation and identification of EF.Of the 43 soil samples collected, 19 (11 from Tenerife, 3 from La Gomera and 5 from La Palma) were processed for EF isolation. EF were detected in 5 of the 19 samples processed (26.3%). All strains were isolated from soils of Tenerife (Table 8), from fields under Integrated Production certification, mostly with drip irrigation and altitude > 100 m. After cross check taxonomic data (morphologic and molecular) a species name was assigned to the EF isolates, that were submitted to the Spanish Collection of Type Cultures (CECT) for deposit, with an assigned, specific code number (Table 9).
| Soil Code | Fungi | Island | Data | Altitude (m) | Irrigation | Certif. crop system |
| TS01 | Lecanicillium sp. | Tenerife | 20/07/2017 | 215 | Drip | Integrated |
| TS04 | Metarhizium sp. | Tenerife | 09/08/2017 | 115 | Drip | Integrated |
| TS04 | Beauveria sp. | Tenerife | 09/08/2017 | 115 | Drip | Integrated |
| TS05 | Lecanicillium sp. | Tenerife | 09/08/2017 | 150 | Drip | Integrated |
| TS08 | Lecanicillium sp. | Tenerife | 20/09/2017 | 135 | Drip | Integrated |
| TS11 | Beauveria sp. | Tenerife | 20/09/2017 | 75 | Sprinkler | Integrated |
Table 8. Entomopathogenic fungi isolated from banana crop soils in the Canary Islands.
| Species | Lab code | CECT number |
| Beauveria bassiana | B.b.1TS11 | CECT 21121 |
| Beauveria bassiana | B.b.19TS04 | CECT 21122 |
| Akanthomyces lecanii | L.l.2TS05 | CECT 21123 |
| Akanthomyces lecanii | L.l.5TS08 | CECT 21124 |
| Akanthomyces lecanii | L.l.6TS01 | CECT 21125 |
| Metarizhium anisopliae | M.a.4TS04 | CECT 21126 |
Table 9. Deposit of EF strains in CECT culture collection.
Total strains isolated were 143 from banana soils of Canary Islands, of which 69 (48.3%) were identified, including Metarhizium, Beauveriaand Lecanicilliumspp. The most common fungi belonged to Fusarium(62.3%), followed by Penicillium(11.6%). The most frequent EF genus isolated was Lecanicilliumsp. (4.3%), followed by Beauveriasp. (2.9%) and Metarhiziumsp. (1.4%). Eleven soil samples (61.1%) were processed from Tenerife, and 264 larvae of G. mellonellaas EF baits were examined. Isolates obtained from Tenerife Island were: 29 Fusariumsp., 4 Penicilliumsp., 2 Cladosporiumsp., 2 Mortierellasp., 3 Lecanicilliumsp., 1 Metarhiziumsp. and 2 Beauveriasp. On the island of Tenerife almost 14% of the fungal isolates were EF, and it was the only island where EF were detected (Table 8). From La Gomera, La Palma and Gran Canaria no EF was isolated.
Morphological identification of EF isolates. Fifteen-day-old colonies from fungal isolates were used for identification with light microscopy. Micro-cultures were also performed from colonies by inoculating isolates in 1´1 cm fragments of CMA on sterile slides, covered with a coverslip and kept in moist chambers to favour formation of reproductive structures easy to identify using a microscope. In this way, isolates were determined up to genus level, using general taxonomic references.
Fungal preservation. EF Isolates were preserved in filter papers following this method: 21-day-old colonies of EF strains on CMA were used as inoculum. A plug (5mm) of EF fresh colony was set in the middle of a CMA petri dish. Then, 5-10 pieces of sterile filter paper (5´5 mm) were placed around the initial inoculum plug. The dishes were then sealed and incubated at 25 ° C in the dark to favour fungal growth. The EF colonized the paper fragments for 20-25 days, that were then harvested axenically and kept in sterile Petri dishes in a desiccator with silica gel for 3-4 days. When filter papers were dry (they produced sound when shaken), they were axenically placed in sterilized envelopes, sealed and labelled with the name of the fungal strain and the code of the soil sampling location, and stored at -20 ° C with silica gel (Fig. 21).
Molecular identification of EF.Besides morphological identification, DNA of the ITS region (internal transcribed spacers) of EF isolates was amplified and sequenced with standard protocols.
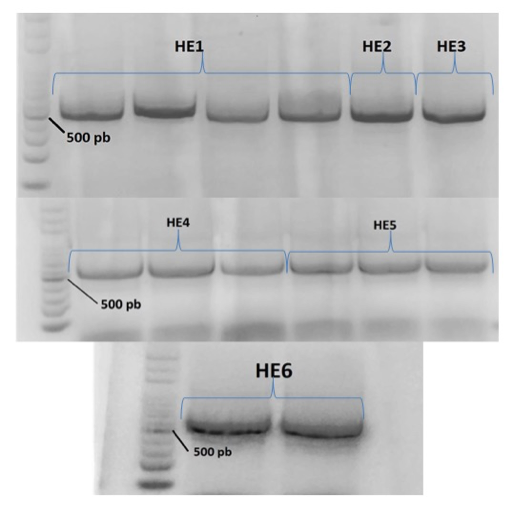
For PCR amplification of the ITS region and sequencing these primers were used. Gel electrophoresis of PCR products showed single bands of ca. 500 bp (Fig. 22). The bands were excised and the DNA was purified for sequencing, for subsequent identification by comparison with sequences available in NCBI, applying coverage and similarity rates of 100% (Table 10).
Sequence analysis using NCBI BLAST.Consensus ITS regions of the fungal strains (product size 500-600 bp) were compared by homology with other sequences in the NCBI database (GeneBank) using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Matching sequences (homology >95%) were used to assign associated species in the NCBI database. The results were crosschecked with light microscopy identifications.
| Sample code | BLASTn | Coverage (%) | Identity (%) | Access No. | Identification | |
| 1 TS11 | Beauveria bassiana | 100 | 100 | KT378236.1 | Beauveria bassiana 1TS11 | |
| 2 TS05 | Simplicillium lamellicola | 100 | 100 | KT004573.1 | Lecanicillium lecanii 2TS05 | |
| 4 TS04 | Metarhizium anisopliae | 100 | 100 | MH483917.1 | Metarhizium anisopliae 4TS04 | |
| 5 TS08 | Simplicillium lamellicola | 100 | 100 | KT004573.1 | Lecanicillium lecanii 5TS08 | |
| 6 TS01 | Simplicillium sp. | 100 | 100 | KY305078.1 | Lecanicillium lecanii 6TS01 | |
| 19 TS04 | Beauveria bassiana | 100 | 100 | MH483769.1 | Beauveria bassiana 19TS04 | |
Table 10. Analysis of ITS sequence homology of EF strains obtained from banana soils compared with NCBI closest accessions.
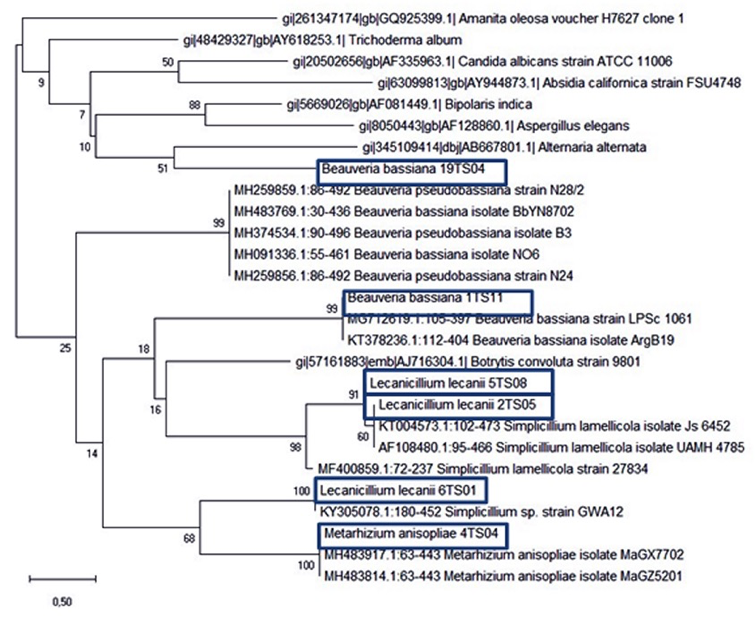
Phylogenetic analysis of sequences. Phylogenetical analyses were performed to confirm molecular identifications of EF strains, with retrived NCBI sequences, compared by the Neighbor join method with more than 1000 comparison using MEGA-X software. Finally a consensus phylogenetic tree was obtained including all the sequences of EF isolates (Fig. 23).
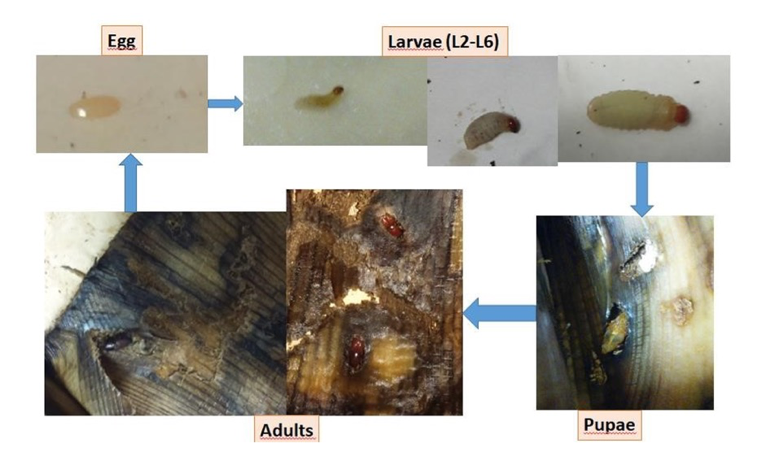
Laboratory rearing of Cosmopolites sordidus. A population of 400 adults of banana weevil (BW, kindly provided by Dr. Ana Piedra-Buena ICIA, Tenerife, Spain), was used to rear the insects aerated plastic boxes at 25-30ºC in the dark, fed with fresh pieces of banana corm and pseudostem (Fig. 24), and kept humid. The decomposed BW diet was eliminated from the rearing boxes weekly. Water containers were also filled weekly. Initially, eggs of BW were set on the surface of the corm and the pseudostem of the banana tree. These were collected weekly and placed on filter paper for incubation and hatching on a sterile filter paper sandwich moistened with SDW. Paper sandwiches with BW eggs were incubated in Petri dishes at 25-30 °C in the dark. Emerging larvae were placed in holes of banana pseudostem fragments to avoid exposure to the environment. BW eggs hatching was scored weekly, using a fine brush moistened to handle the eggs and early larval stages, to avoid damage. For insect maintenance, corm and pseudostem pieces were checked for BW adults, pupae, larvae and eggs every 20-30 days. BW larvae and pupae were placed in new breeding boxes with fresh banana corm and water to continue their development. BW Adults (ca. 50 individuals) were placed per rearing box with fresh diet and water.

Only banana corm allowed BW to complete its biological cycle (from egg to adult, Fig. 25). Therefore, the corm and pseudostem were chosen as a diet for rearing. BW egg hatching on filter paper was 18% (19 L1 / 105 H). The L1 larvae did not survive handling when placed in fresh corm pieces and banana pseudostem.
UA team obtained new BW adults from eggs deposited in pieces of corm or pseudostem of banana tree by the original BW adult (Table 11). Viable larvae were only obtained when large pieces of corm were placed in the rearing boxes. Therefore, BW laboratory rearing is slow and requires large amounts of fresh banana material.
| Date | Eggs | Larvae (L2-L6) | Pupae | Adults |
| 10-jan | – | 9 | – | – |
| 15-jan | – | 5 | – | – |
| 23-jan | 6 | – | – | – |
| 29-jan | 55 | – | – | – |
| 01-feb | – | – | – | – |
| 05-feb | 14 | 2 | 1 | 3 |
| 07-feb | – | – | – | 1 |
| 14-feb | – | – | – | – |
| 22-feb | 10 | – | – | – |
| 06-mar | – | – | – | – |
| 12-mar | 20 | 69 | 1 | 1 |
| TOTAL | 105 | 85 | 2 | 5 |
Table 11. Data on Cosmopolites sordidus laboratory rearing (January-March, 2018).
Pathogenicity assays. Six strains of EF isolated from Canary Islands were used for pathogenicity bioassays to check virulence (2 B. bassiana, 1 M.anisopliaeand 3 L. lecanii). EF pathogenicity was evaluated by testing survival of G. mellonellalarvae inoculated with a given strain of EF under lab conditions. Further strains from the Fungal Collection of Plant pathology Laboratory at University of Alicante were also used (Table 12), to compare the virulence of isolates from Tenerife soils with that of EF from other areas.
| Isolate | Host | Origin |
| Beauveria bassiana 203 | Rhynchophorus ferrugineus, red palm weevil (Coleoptera) | Daimés (Elche, SE Spain) |
| Beauveria bassiana 53 | Rhizotrogus chevrolati (Coleoptera) | Setúbal (Portugal) |
| Beauveria bassiana 119 | Langia sp. (Coleoptera) | Orihuela (Alicante, SE Spain) |
| Metarhizium anisopliae 46 | Otiorhynchus sulcatus (Coleoptera) | – |
| Lecanicillium cf. psaliotae | Phoenicococcus marlatti(red palm scale) | Elche (Alicante, SE Spain) |
| Lecanicillium lecanii 131 | G. mellonella | Alicante (SE Spain) |
Table 12. EF from Plant pathology Laboratory of the University of Alicante Fungal Collection.
Living larvae of G. mellonellawere inoculated with the above described isolates placing five larvae (L3-L4) in 9 cm dishes with a 24-day colony of each EF isolate grown in CMA. Insects were kept on the plate for 5 min, with regular shaking to favour contact with the fungus. For controls, plates with non-inoculated CMA medium were used. Insects were placed in 9 cm Petri in a moist chamber to determine the time in which the fungus kills the larvae by evaluating mortality every 12 hours (Fig. 26). A similar experiment with no added moisture (environmental humidity) was also carried out and repeated once.

Pathogenicity bioassays were performed by placing in contact G. mellonellalarvae with a 15-day-old colony of EF grown in CMA. In the pathogenicity tests, EF isolated from banana crop soils were compared with similar EF strains from the Plant Pathology Laboratory Collection (University of Alicante). We observed that the most virulent strain was B. bassiana1TS11 from Tenerife, followed by B. bassiana19TS04 and M. anisopliae4TS04, from Tenerife and three fungi from the UA collection (B. bassiana203, B.bassiana53 and M. anisopliae46). These fungi generatedG. mellonella100% mortality after two days (Fig. 27). In the second replicate of this test the same virulence was observed. In the experiments at environmental humidity, the results were similar to the moist chamber ones, with a delay to achieve 100% mortality but with the same pathogenic characteristics of the strains. This suggests that EF strains from banana fields are active under environmental stress (low humidiy).

Moist chambers tests data showed that B. bassiana 19TS04, despite having more inoculum per larva (1.69E+06±5.59E+05) than B. bassiana 1TS11 (1.23·106 ± 4.45·105) was significantly less pathogenic, with 1.8 and 1.3 estimated survival mean times, respectively, indicating that 50% of individuals were dead at those times. M. anisopliae 4TS04 having a lower inoculum (2.13·105± 1.96·105) than B. bassiana 19TS04, had no effect on the cumulative survival rate (no significant differences). Finally, L. lecanii 2TS05 and L. lecanii 5TS08 treated larvae did not differ from the uninoculated ones, indicating a lower pathogenicity of both isolates to G. mellonella(Table 13).
| Isolate | Environmental Humidity | Moist chamber | |||
| Spores (·106mean ±SE) | Estimated survival mean time (days) | Spores (mean ±SE) | Estimated survival mean time (days) | ||
| Lecanicillium lecanii 131 | 0.402 ± 0.404 | 15.3 | 0.327 ± 0.0575 | 13.033 | |
| Lecanicillium psaliotae | 0.712 ± 0.555 | 15.133 | 0.162 ± 0.097 | 5.4 | |
| Beauveria bassiana 203 | 9.08 ± 5.46 | 4.5 | 0.218± 0.0407 | 1.767 | |
| Beauveria bassiana 53 | 1.51 1.73 | 6.133 | 0.255 ± .163 | 2.167 | |
| Beauveria bassiana 119 | 0.0423± 0.011 | 6.533 | 10000 | 2.393 | |
| Metarhizium anisopliae 46 | 2.22 ± 1.06 | 5.467 | 0.173 ± 0.142 | 1.733 | |
| Beauveria sp. 19TS04 | 6.96± 4.62 | 4.714 | 1.69 ± 0.559 | 1.821 | |
| Beauveria sp. 1TS11 | 11.0 ± 10.2 | 4.75 | 1.23 ± 0.445 | 1.367 | |
| Metarhizium sp. 4TS04 | 0.808 ± 0.697 | 4.714 | 0.213 ± 0.196 | 2 | |
| Lecanicillium sp. 5TS08 | 15.2 ± 9.03 | 11.607 | 0.117 ± 0.244 | 11.467 | |
| Lecanicillium sp. 2TS05 | 4.54± 0.865 | 10.714 | 1.94 ± 0.961 | 9.967 | |
| Lecanicillium sp. 6TS01 | 2.70 ± 1.71 | 7.893 | 1.53 ± 1.12 | 8.6 | |
| Control | – | 15.864 | – | 14.267 | |
Table 13. Number of spores adhered to G. mellonella larvae used in the virulence tests in moist chambers and under environmental humidity and the estimated survival mean time (time in days when 50% of the larvae are dead) in each incubation (with and without humidity) condition.
UA also performed a pathogenicity test on Cosmopolites sordidus using larvae of sizes comprised between 0.5 and 2 cm (included several larval stages), that were very sensitive to handling and dryness. The larval mortality was very high in the first hours, even for control larvae (exposed to uninoculated CMA). Even so, Metarhiziumsp. 4TS04 was slightly, although not significantly, more virulent than Beauveria sp. 19TS04 with 87% and 69% mortality after 1.5 days, respectively. In the control, the mortality at 1.5 days was 60%. Both EF achieved full mortality (100%) at 2.5 days while controls achieved this level at 6 days.
Entomopathogenic nematodes. Lines of entomopathogenic nematodes (EPN) (Fig. 28) were obtained at CENSA by the baiting technique, using healthy late instar larvae of the greater wax moth Galleria mellonella(L.) (Lepidoptera: Pyralidae), obtained from a laboratory culture. The EPN were maintained using cycles of infection of G. mellonella larvae. The juveniles were kept in clear nylon envelopes using sponges at 22ºC under laboratory conditions, and identified by a code (Fig. 29).
The G. mellonella cadavers from all the isolates were dark brown, suggesting the presence of the genus Heterorhabditis. From the second cycle of infection, some G. mellonella cadavers were dissected under a stereomicroscope on the fourth day and the hermaphrodite adults were observed under a microsope, confirming their identity as Heterorhabditis.


Forin vitro pathogenicity assays of HC1 strain of H. amazonensis against BW adults (1st trial) two types of traps were evaluated to obtain BW adults. The traps used at the farm Los González were the Sandwich trap and the disc trap. The disc trap showed best results, and was recommended for BW collection (Fig. 30).
BW adults with similar weight and size, taken from a quarantine cabinet (Fig. 31), were used in EPNs trial. The BW mortality was daily evaluated from 12 hours to 21 days. The dead BWs were placed (individually) in a Petri dish and dissected after 4-5 days, and the hermaphrodite adults were observed. A mortality around 86.7 % was obtained with the highest EPN dose, after 21 days, significantly higher (R² = 0.78) than control.

Antagonistic effect of endophytic bacteria.IITA developed a number of pot and field assays to check the ability of a number of available isolates in management of BW and/or PPN. In vitroassays to test potential of newly isolated banana endophytes to control Foc race 1. Isolates of endophytic bacteria were tested using the dual culture method in the laboratory. Preliminary results from testing of 100 pure potential endophytic bacteria identified 11 isolates that significantly reduced growth of Foc race 1 under in vitroconditions. This is an ongoing activity and testing of the remaining 270 isolates from Tanzania and all isolates from Uganda is in progress.
Testing efficacy of previously isolated fungal isolates for managing nematodes and weevils.These studies are underway in Uganda, using two fungal isolates: non-pathogenic Fusarium oxysporumisolate V5w2 and Beauveria bassianaisolate GA. The F. oxysporumisolate was originally isolated from roots of healthy banana plants in Uganda and preserved in sterile glycerol in Eppendorf tubes at -80°C at the Department of Plant Pathology, Stellenbosch University, Stellenbosch, South Africa. While the B. bassiana isolate was originally isolated from a mycosed banana weevil (Cosmopolites sordidus) in Uganda, freeze-dried and preserved at the culture collection unit of CABI, UK. Both fungal isolates were provided as actively growing cultures on agar plates from the respective institutes, following correct procedures for importation. Cultures were then multiplied on rice grains in Uganda for experimentatal purposes.
Pot experiments. A pot experiment was set up using in vitrocooking banana plantlets cv. Mbwazirume (AAA-EA). The plantlets were inoculated with the fungal endophytes Fusarium oxysporum(V5w2) and Beauveria bassiana (GA). The fungi were inoculated onto banana plantlets individually and in combination to assess compatibility. The plantlets were later challenged with the burrowing nematode Radopholus similisand banana weevil (1:1, F:M). Results from this preliminary experiment indicate that inoculation of banana plantlets with the fungal endophytes reduced nematode levels to 198 and 211 nematodes/5 g root weight forF. oxysporumand B. bassianainoculated plants respectively, compared to 323 nematodes recovered per 5 g of root in the control plants. Furthermore, inoculating banana plantlets with a combined inoculum of both F. oxysporumand B. bassianagreatly reduced nematode population to 33 nematodes / 5 g of root. Colonization of the banana rhizome by the fungal endophytes was estimated at 58% and 47% for B. bassianaand F. oxysporumrespectively with no apparent penalty to plant growth. The average percentage BW damage to the banana rhizome was estimated at 50%, 40%, 71% and 45% for plants inoculated with B. bassiana, a combination of B. bassiana+ F. oxysporum, F. oxysporumand the control plants respectively. Fungal colonization of the banana roots was estimated at 37% and 43%, while colonization of the banana rhizome was estimated at 58 % and 47% forB. bassianaand F. oxysporumrespectively.
Following these preliminary results, follow up pot trials were established to further assess efficacy of these two fungal endophytes in isolation and in combination, to target: 1) single nematode species, 2) mixed nematode species, 3) mixed nematode and banana weevil and 4) banana weevil infestations.
Task 2.4 – Host range assessment and plant receptivity assays
Evaluation of selected EBCAs vs PD in pot-grown cv Gran Enana.EBCAs isolated by CNR from banana rhizosphere soil collected in Tenerife, including fungi such as Trichodermaand bacteria such as Streptomyces, Pseudomonasand Bacillus, were used to set up a synthetic microbial community (SynCom), tested in plantain consecutive experiments against Foc. The SynCom (version 1.0) was constructed with 44 selected beneficial microorganisms derived from banana rhizosphere, of genera Streptomyces, Bacillus, Pseudomonasand Trichoderma(see Table 3). In experiments I and II, the PD symptoms appeared 14 days post-inoculation (dpi) and increased afterwards. Both versions of SynCom (1.0 and 1.1) were fully compatible with (not deleterious to) Gran Enana plants, and their inoculation partially reduced PD severity.
Interactions between endophytes and plants.In Gran Enana soil and rhizosphere, the populations of the EBCAs composing the SynCom remained viable and active throughout the experiment, although they did not regulate the Foc TR4 population density.A total of 9 Trichodermaspp. were selected in Costa Rica by EARTHfor greenhouse evaluation as EBCAs. The best isolates were compatible with the Cavendish germplasm tested after introduction in banana fields, for preliminary assays against R. similisand other PPN. Based on results from previous work (Del. 2.2) a bioassays was implemented to compare the four best isolates with available commercial products (Soilset™ and Agromos™) used as plant growth promotors. Results showed that the four endophytes selected in WP2 performed better than the commercial products.